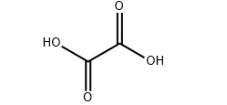
 Chemical Properties: Oxalic acid is widely distributed in the plant in nature, most existing in the form of oxalic acid salt. C.W. Scheele had for the first time manufactured oxalate in 1776. Oxalate is the strongest acid among the dicarboxylic acid. Besides having the general properties of the carboxylic acid, it also has reducing property and can quantitatively reduce the seven valence manganese to bivalent manganese. This property is often used for quantitative analysis of potassium permanganate. 5 C2H2O4 + 2 KMnO4 + 3 H2SO4 →K2SO4 + 2 MNSO4 + 8H2O + 10 CO2; Oxalic acid can also reduce the trivalent iron into bivalent iron. Because of the high solubility of the bivalent iron in the water, we can apply this principle to remove rust on the clothes. Oxalic acid can react with phosphorus pentachloride to generate phosphorus oxychloride. C2H2O4 + PCl5 → POCl3 + CO + CO2 + 2 HCL. Oxalic acid can react with many metals to produce oxalic acid salt. In addition to the alkali metal salt and bivalent iron salts with the rest of the oxalic acid salt being poorly soluble in water. Some metal salt, although is poorly soluble in water, can generate complex that is soluble in water. Fe2 (C2O4) 3 + 3 K2C2O4 + 6 H2O →2 K3 [Fe (C2O4) 3] • 6 H2O. Upon heating, alkali metal and alkaline earth metal oxalic acid salt can lose carbon monoxide and form carbonates with carbonate continuing to be subject to heating to be further decomposed into oxide and carbon dioxide. The oxalic acid salt of nickel, cobalt and silver can finally produce metal instead of nonmetal oxide. The decomposing products of the oxalate are carbon dioxide, carbon monoxide and water. Oxalate and oxalic acid salt are toxic. Mice, through oral administration, has LD50 of 2000~4000 mg/kg. Application: 1. Oxalic acid can be mainly used as reducing agent and bleaching agent, mordant for dyeing and printing industry, also used in refining rare metal, the synthesis of various oxalate ester amide, oxalate and grass, etc. 2. Used as analytical reagent. 3. Used as laboratory reagents, chromatography analysis reagent, dye intermediates and standard material. 4. Oxalic acid is mainly used for producing drugs such as antibiotics and borneol and solvent for extracting the rare metal, reducing agent and dye, tanning agent, etc. In addition, oxalic acid can also be used for the synthesis of various kinds of oxalate ester, oxalate, and oxamide with diethyl oxalate, sodium oxalate and calcium oxalate having the largest yield. Oxalate can also be used for the production of cobalt-molybdenum-alumina catalyst, cleaning of metal and marble as well as the bleaching of textiles.
Chemical Properties: Oxalic acid is widely distributed in the plant in nature, most existing in the form of oxalic acid salt. C.W. Scheele had for the first time manufactured oxalate in 1776. Oxalate is the strongest acid among the dicarboxylic acid. Besides having the general properties of the carboxylic acid, it also has reducing property and can quantitatively reduce the seven valence manganese to bivalent manganese. This property is often used for quantitative analysis of potassium permanganate. 5 C2H2O4 + 2 KMnO4 + 3 H2SO4 →K2SO4 + 2 MNSO4 + 8H2O + 10 CO2; Oxalic acid can also reduce the trivalent iron into bivalent iron. Because of the high solubility of the bivalent iron in the water, we can apply this principle to remove rust on the clothes. Oxalic acid can react with phosphorus pentachloride to generate phosphorus oxychloride. C2H2O4 + PCl5 → POCl3 + CO + CO2 + 2 HCL. Oxalic acid can react with many metals to produce oxalic acid salt. In addition to the alkali metal salt and bivalent iron salts with the rest of the oxalic acid salt being poorly soluble in water. Some metal salt, although is poorly soluble in water, can generate complex that is soluble in water. Fe2 (C2O4) 3 + 3 K2C2O4 + 6 H2O →2 K3 [Fe (C2O4) 3] • 6 H2O. Upon heating, alkali metal and alkaline earth metal oxalic acid salt can lose carbon monoxide and form carbonates with carbonate continuing to be subject to heating to be further decomposed into oxide and carbon dioxide. The oxalic acid salt of nickel, cobalt and silver can finally produce metal instead of nonmetal oxide. The decomposing products of the oxalate are carbon dioxide, carbon monoxide and water. Oxalate and oxalic acid salt are toxic. Mice, through oral administration, has LD50 of 2000~4000 mg/kg. Application: 1. Oxalic acid can be mainly used as reducing agent and bleaching agent, mordant for dyeing and printing industry, also used in refining rare metal, the synthesis of various oxalate ester amide, oxalate and grass, etc. 2. Used as analytical reagent. 3. Used as laboratory reagents, chromatography analysis reagent, dye intermediates and standard material. 4. Oxalic acid is mainly used for producing drugs such as antibiotics and borneol and solvent for extracting the rare metal, reducing agent and dye, tanning agent, etc. In addition, oxalic acid can also be used for the synthesis of various kinds of oxalate ester, oxalate, and oxamide with diethyl oxalate, sodium oxalate and calcium oxalate having the largest yield. Oxalate can also be used for the production of cobalt-molybdenum-alumina catalyst, cleaning of metal and marble as well as the bleaching of textiles. Products Details
 Chemical Properties: Oxalic acid is widely distributed in the plant in nature, most existing in the form of oxalic acid salt. C.W. Scheele had for the first time manufactured oxalate in 1776. Oxalate is the strongest acid among the dicarboxylic acid. Besides having the general properties of the carboxylic acid, it also has reducing property and can quantitatively reduce the seven valence manganese to bivalent manganese. This property is often used for quantitative analysis of potassium permanganate. 5 C2H2O4 + 2 KMnO4 + 3 H2SO4 →K2SO4 + 2 MNSO4 + 8H2O + 10 CO2; Oxalic acid can also reduce the trivalent iron into bivalent iron. Because of the high solubility of the bivalent iron in the water, we can apply this principle to remove rust on the clothes. Oxalic acid can react with phosphorus pentachloride to generate phosphorus oxychloride. C2H2O4 + PCl5 → POCl3 + CO + CO2 + 2 HCL. Oxalic acid can react with many metals to produce oxalic acid salt. In addition to the alkali metal salt and bivalent iron salts with the rest of the oxalic acid salt being poorly soluble in water. Some metal salt, although is poorly soluble in water, can generate complex that is soluble in water. Fe2 (C2O4) 3 + 3 K2C2O4 + 6 H2O →2 K3 [Fe (C2O4) 3] • 6 H2O. Upon heating, alkali metal and alkaline earth metal oxalic acid salt can lose carbon monoxide and form carbonates with carbonate continuing to be subject to heating to be further decomposed into oxide and carbon dioxide. The oxalic acid salt of nickel, cobalt and silver can finally produce metal instead of nonmetal oxide. The decomposing products of the oxalate are carbon dioxide, carbon monoxide and water. Oxalate and oxalic acid salt are toxic. Mice, through oral administration, has LD50 of 2000~4000 mg/kg. Application: 1. Oxalic acid can be mainly used as reducing agent and bleaching agent, mordant for dyeing and printing industry, also used in refining rare metal, the synthesis of various oxalate ester amide, oxalate and grass, etc. 2. Used as analytical reagent. 3. Used as laboratory reagents, chromatography analysis reagent, dye intermediates and standard material. 4. Oxalic acid is mainly used for producing drugs such as antibiotics and borneol and solvent for extracting the rare metal, reducing agent and dye, tanning agent, etc. In addition, oxalic acid can also be used for the synthesis of various kinds of oxalate ester, oxalate, and oxamide with diethyl oxalate, sodium oxalate and calcium oxalate having the largest yield. Oxalate can also be used for the production of cobalt-molybdenum-alumina catalyst, cleaning of metal and marble as well as the bleaching of textiles.
Chemical Properties: Oxalic acid is widely distributed in the plant in nature, most existing in the form of oxalic acid salt. C.W. Scheele had for the first time manufactured oxalate in 1776. Oxalate is the strongest acid among the dicarboxylic acid. Besides having the general properties of the carboxylic acid, it also has reducing property and can quantitatively reduce the seven valence manganese to bivalent manganese. This property is often used for quantitative analysis of potassium permanganate. 5 C2H2O4 + 2 KMnO4 + 3 H2SO4 →K2SO4 + 2 MNSO4 + 8H2O + 10 CO2; Oxalic acid can also reduce the trivalent iron into bivalent iron. Because of the high solubility of the bivalent iron in the water, we can apply this principle to remove rust on the clothes. Oxalic acid can react with phosphorus pentachloride to generate phosphorus oxychloride. C2H2O4 + PCl5 → POCl3 + CO + CO2 + 2 HCL. Oxalic acid can react with many metals to produce oxalic acid salt. In addition to the alkali metal salt and bivalent iron salts with the rest of the oxalic acid salt being poorly soluble in water. Some metal salt, although is poorly soluble in water, can generate complex that is soluble in water. Fe2 (C2O4) 3 + 3 K2C2O4 + 6 H2O →2 K3 [Fe (C2O4) 3] • 6 H2O. Upon heating, alkali metal and alkaline earth metal oxalic acid salt can lose carbon monoxide and form carbonates with carbonate continuing to be subject to heating to be further decomposed into oxide and carbon dioxide. The oxalic acid salt of nickel, cobalt and silver can finally produce metal instead of nonmetal oxide. The decomposing products of the oxalate are carbon dioxide, carbon monoxide and water. Oxalate and oxalic acid salt are toxic. Mice, through oral administration, has LD50 of 2000~4000 mg/kg. Application: 1. Oxalic acid can be mainly used as reducing agent and bleaching agent, mordant for dyeing and printing industry, also used in refining rare metal, the synthesis of various oxalate ester amide, oxalate and grass, etc. 2. Used as analytical reagent. 3. Used as laboratory reagents, chromatography analysis reagent, dye intermediates and standard material. 4. Oxalic acid is mainly used for producing drugs such as antibiotics and borneol and solvent for extracting the rare metal, reducing agent and dye, tanning agent, etc. In addition, oxalic acid can also be used for the synthesis of various kinds of oxalate ester, oxalate, and oxamide with diethyl oxalate, sodium oxalate and calcium oxalate having the largest yield. Oxalate can also be used for the production of cobalt-molybdenum-alumina catalyst, cleaning of metal and marble as well as the bleaching of textiles. Featured Products
- Styrene (SM) CAS 100-42-5 High Quality And Low Price
- Sodium Tripolyphosphate (STPP) CAS 7758-29-4 China Best Price
- 1-Octanol CAS 111-87-5 High Quality And Low Price
- petroleum resin CAS 68131-77-1 High Quality And Low Price
- Acrylonitrile (AN) CAS 107-13-1 Factory Direct Supply
- Acetic Acid CAS 64-19-7 China Best Price
- Tetrahydrofuran (THF) CAS 109-99-9 High Quality And Low Price
- Acetone CAS 67-64-1 China Best Price
- 2-tert-Butylphenol CAS 88-18-6 Factory Direct Supply
- Methyl Methacrylate (MMA) CAS 9011-14-7 China Best Price
Contact us
Please feel free to give your inquiry in the form below We will reply you in 24 hours